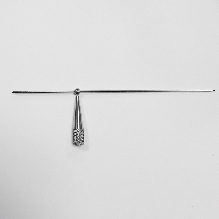
Threaded pins with Anti-Migration ends
Product description
Both the threaded pins and anti-migration ends are manufactured from ISO 5832-1 standard 316L stainless steel. All materials are sourced from ISO 9000 certified suppliers. Each raw material delivery comes with a certificate of origin of the material.
The threaded pins and anti-migration ends are produced in accordance with our manufacturing" procedures: a manufacturing order is issued by the Production Manager. For each manufacturing order, the Production Manager assigns a four digit batch number. The manufacturing order details all the operations to be carried out on the products. At each stage, various dimensional verifications are carried out by the manufacturing operators and by the Production Manager. All inspection forms are kept with the manufacturing order.
Dimensional characteristics
The threaded pins and anti-migration ends have the following dimensional characteristics:
- 38.63.13 / 18 : 1 mm diameter threaded pin + anti-migration end
- 38.63.14 / 18 : 1.2 mm diameter threaded pin + anti-migration end
- 38.63.15 / 19 : 1.5 mm diameter threaded pin + anti-migration end
- 38.63.16 / 20: 1.8 mm diameter threaded pin + anti-migration end
- 38.63.17 / 20: 2 mm diameter threaded pin + anti-migration end
PIN WITH ANTI-MIGRATION BALL
5 types available: Ø 1mm( 38.63.13 / 38.63.18) - Ø 1.2mm ( 38.63.14 / 38.63.18) - Ø 1.5mm ( 38.63.15 / 38.63.19) -
Ø 1.8mm ( 38.63.16 / 38.63.20) and Ø 2mm (38.63.17 / 38.63.20)
Indications of use: Bone fractures of the hand, wrist, foot or ankle
Surgical technique:
On opening the peel & seal bag, put to one side the traceability stickers (1 for order renewal, 4 for the patient file)
* On inserting the pin, the ball is on the pin without blocking position.
* Cut the pin.
* Screw the ball mount until the screw breaks off.
To ensure traceability, the threaded pins and anti-migration ends are individually packaged in double blister packs, the first containing the threaded pin and anti-migration end and a sterilisation indicator label. The second blister contains the first blister and five traceability stickers with the screw reference, description and batch number.
The packed products are then sent to ISOTRON FRANCE SA for sterilisation by gamma radiation according to the ISO 11137-02, ISO 11737-1, ISO 11737-2, EN 552, EN 556 standards and dose mapping. After sterilisation, ISOTRON returns the batch of sterilised products with a certificate of sterilisation by gamma radiation.
The pins in the packaging are then labelled. The label includes the description, the reference, the production batch number, the method of sterilisation, the sterilisation batch number, expiry date, the manufacturer's name (CHOC) and the CE 0499 markings.
The threaded pins and anti-migration ends are packed and stored in closed cupboards, away from direct light.