CHOCAFIX®
Product description
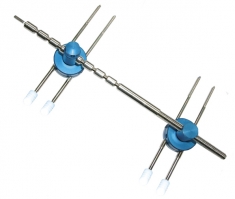
CHOCAFIX® external fixator system for the wrist.
CHOCAFIX® includes a fixator module, 4 threaded pins and four protective pin ends.
It comes sterilized, for single use with five traceability stickers.
CHOCAFIX® is recommended for all fractures to the extremity of the radius and/or ulna at the wrist.
Dimensional characteristics
A CHOCAFIX system is comprised of a module + four pins + four ends.
14.63.04 External fixator rod CHOCAFIX
14.63.23 Pin ends protectors CHOCAFIX
14.33.63 External fixator mounts CHOCAFIX
CHOCAFIX does not require any particular ancillary equipment; an adjustment key is provided and the pins are screwed in using the motor unit.
Fitting technique
Unscrew the 2 pin mounts and separate the threaded parts.
Screw the first orifice of the threaded part on the first pin and the second on the second pin.
Fix the mount rod by re-screwing the mounts on the threaded parts.
The smooth orifices are used as a drilling template for the third or fourth pin.
A slight tightening of the mount rod provides stability of the whole unit in the 3 planes.
Postoperative
Any distraction or compression operation can be carried out at the postoperative follow-up appointment using the adjustment key. The pins are usually extracted 6 to 8 weeks after fitting.
To ensure CHOCAFIX traceability, it comes individually packed in a blister pack comprised of the module, 4 pin ends, 4 pins, an adjustment key and a sterilisation indicator label. This blister is placed in a peel & seal bag with five traceability stickers giving the production reference, description and batch number.
The FIXADYN is then sent to Synergy Health France (MIN des Arnavaux- 13014 MARSEILLE) for sterilisation by gamma radiation according to the ISO 11137-02, ISO 11737-1, ISO 11737-2, EN 552, EN 556 standards and dose mapping. After sterilisation, SYNERGY HEALTH returns the batch of sterilised screws with a certificate of sterilisation by gamma radiation.
The products in the packaging are then labelled. The label includes the description, the reference, the production batch number, the method of sterilisation, the expiry date, the "single use" and "read instructions before use" symbols, the manufacturer's name and address and the CE 1014 markings.
Storage conditions: no special conditions.