FIXADYN®
Product description
FIXADYN® is a dynamic external fixator for the treatment of digital joint fractures.
FIXADYN® is a concept created by Doctors Philippe PASQUIER & Franck DUTEILLE.
It comes sterilised in double blister packs with five traceability stickers.
Dimensional characteristics
FIXADYN® is a kit comprised of the fixator frame (2 distraction plates), two Ø 1.2 mm threaded pins, and four pin ends.
14.63.25 FIXADYN threaded pin
14.63.26 FIXADYN protective pin end
14.33.65 FIXADYN external fixator frame
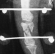
Partial fractures of the base of P2
P2 base fractures caused by compression
Non synthesizable joint fractures of P1
Operating technique - Step 1
Placing of the distractor under locoregional anaesthesia, or even local anaesthesia.
The 2 pins (provided in the kit) must be positioned either side of the joint in question using the pin paralleling tool.
Operating technique - Step 2
The support plates must be placed on the transfixing pins, the cylinder on the more proximal pin.
The cylinder can be placed inside or out.
For static support, for which only a fracture alignment is required without digital mobilisation, it is possible to block the cylinders on the proximal pin by tightening the Allen screws.
For dynamic support, for which joint mobilisation is required, it is best not to block the cylinder on the proximal pin in order to prevent unintentional rotational movement of the pin, which can cause osteolysis.
In the latter case, the distraction plates are laterally fixed by the inserted pin ends.
To ensure FIXADYN traceability, it comes individually packed in a double blister pack with 4 pin ends, 2 pins and a sterilisation indicator label. The second blister contains the first blister and five traceability stickers with the product reference, description and batch number. The plates and elastic bands are supplied decontaminated and unsterilized.
The FIXADYN (blister) is then sent to Synergy Health France (MIN des Arnavaux- 13014 MARSEILLE) for sterilisation by gamma radiation according to the ISO 11137-02, ISO 11737-1, ISO 11737-2, EN 552, EN 556 standards and dose mapping. After sterilisation, SYNERGY HEALTH returns the batch of sterilised screws with a certificate of sterilisation by gamma radiation.
The products in the packaging are then labelled. The label includes the description, the reference, the production batch number, the method of sterilisation, the expiry date, the "single use" and "read instructions before use" symbols, the manufacturer's name and address and the CE 1014 markings.
FIXADYN is supplied in a box containing 1 sterile double blister pack (pins + pin ends) and an unsterile bag (plates + elastic bands) identically as above, stretch-wrapped and stored in closed cupboards, away from direct light and humidity.
Storage conditions: no special conditions.